CFD software is no longer a novelty in the pharmaceutical industry, it is used as an essential part of day-to-day business. But Lattice Boltzmann simulations are novel.
While they offer ease of set up, require less computational power, and provide better results for transient schemes, Lattice Boltzmann large eddy simulations (LB LES) still need literature validation in the industry. That’s what this research—with Boehringer Ingelheim and the Institute of Multiphase Flows—aims to resolve, one study at a time.
CFD software has become an essential component of a pharmaceutical manufacturer’s toolkit for scale-up, process design, and optimization. Proven to address issues in up- and downstream development and overall biopharmaceutical production, CFD software reduces costs and improves equipment and process understanding, ultimately leading to a faster time to market.
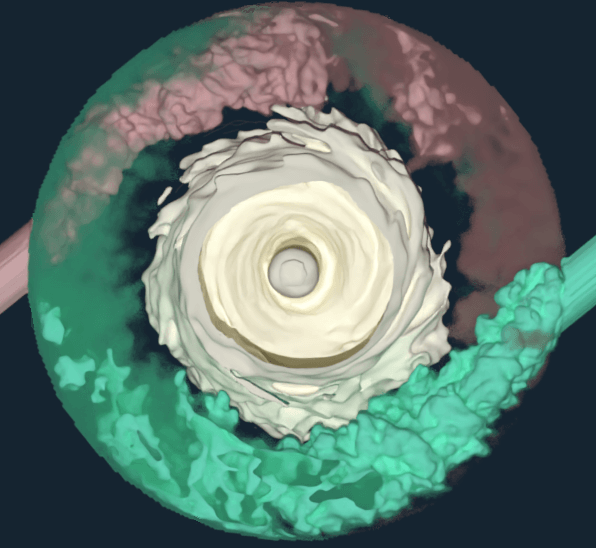
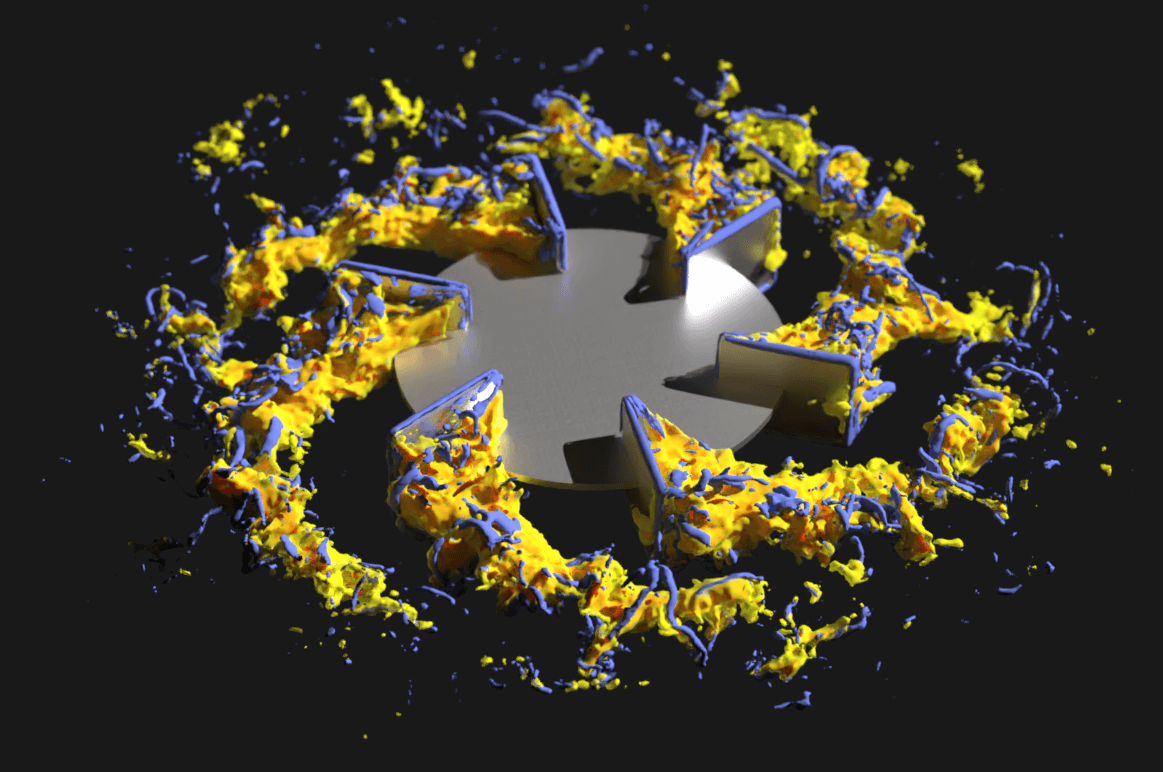
This detailed process and equipment knowledge is critical to the successful development of biopharmaceuticals. In order to get this information, however, simulations require good resolution into the transient structures within stirred tank bioreactors—characterized by their superior combination of mixing performance, mass transfer, and heat transfer.
While the Reynolds-averaged Navier-Stokes (RANS) approach has been extensively reviewed and used in the industry for fast equipment characterization when it comes to power number determination, the approach’s limitations with transient schemes are also well documented. That’s why more and more industry practitioners are turning to LB methods, due to the availability of new commercial solvers like M-Star CFD, which offer more detailed insights into flow patterns for mixing time simulations.
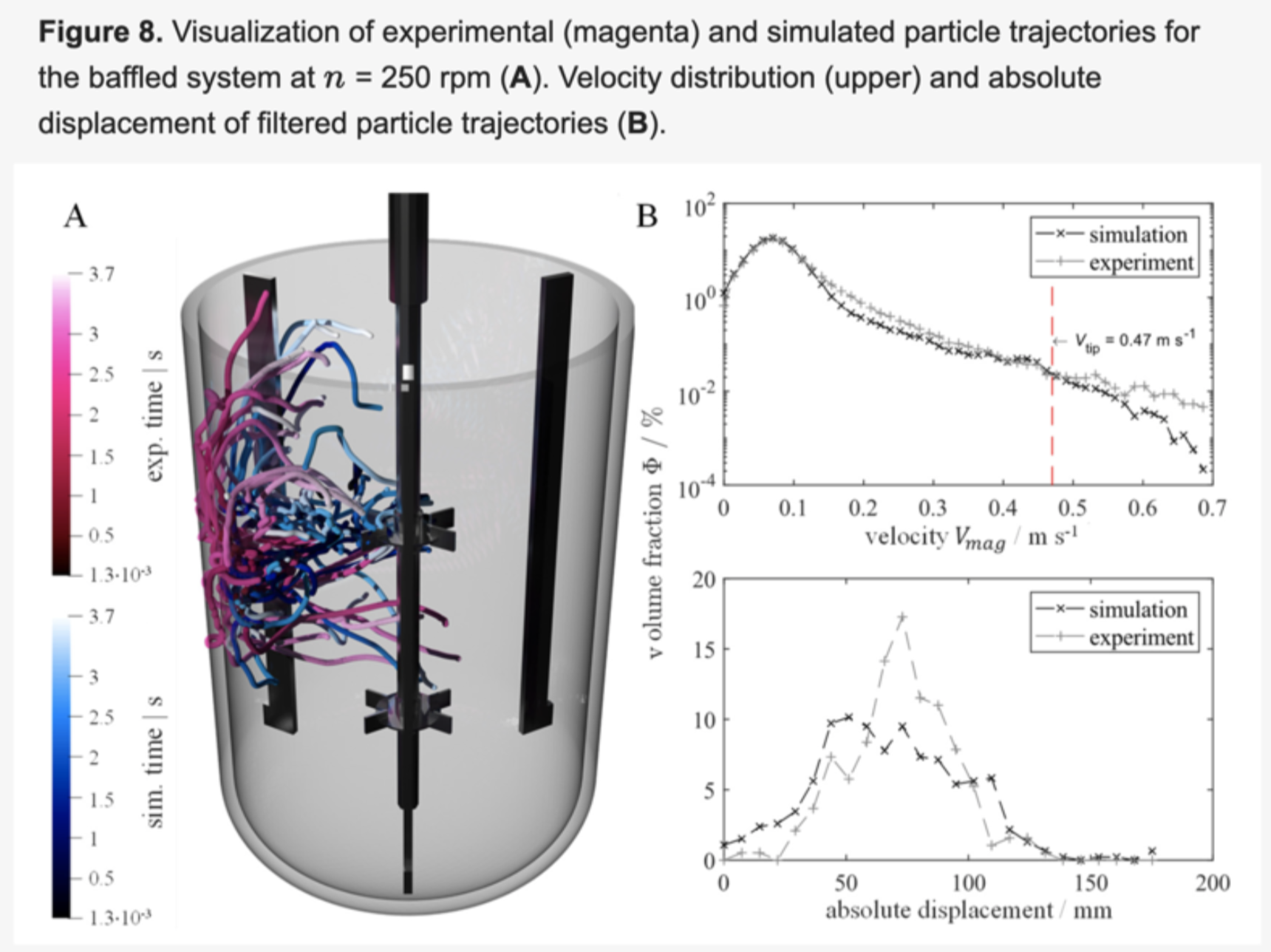
This study seeks to justify the approach to large industrial scales. In this paper, Boehringer Ingelheim (BI) and the Institute of Multiphase Flows use M-Star CFD to apply transient LB LES to a 3L bioreactor system. Then they compare those results to a steady RANS simulation as well as 4D particle tracking (PTV) experiments. The LB LES simulation is shown to be applicable for reliable and detailed prediction of mixing times.
In the full paper, BI and the Institute of Multiphase Flows:
- present full background on the use of simulation models to characterize equipment in the pharmaceutical industry.
- apply LB LES to a 3L bioreactor system.
- validate the approach by comparing LB LES results against steady RANS simulations and 4D PTV experiments.
Read the full paper to learn more about the development of the novel approach and see the industry practical results.
Validation of Novel Lattice Boltzmann Large Eddy Simulations (LB LES) for Equipment Characterization in Biopharma Processes (2021)
Maike Kuschel, Jürgen Fitschen, Marko Hoffmann, Alexandra von Kameke, Michael Schüter, and Thomas Wucherpfennig
 Explore the Scientific R&D Software
Explore the Scientific R&D Software