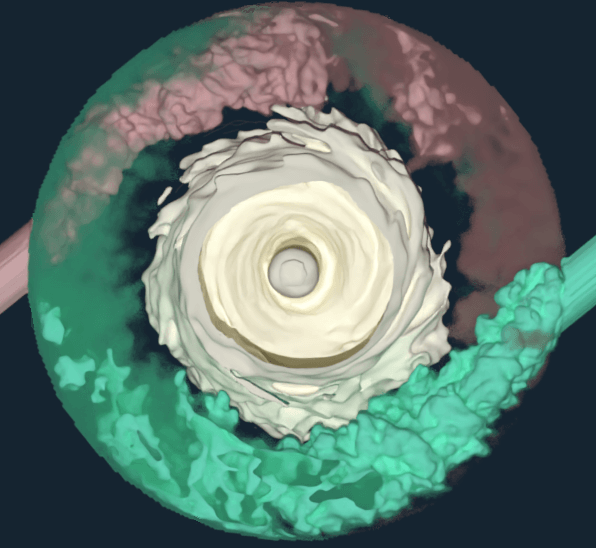
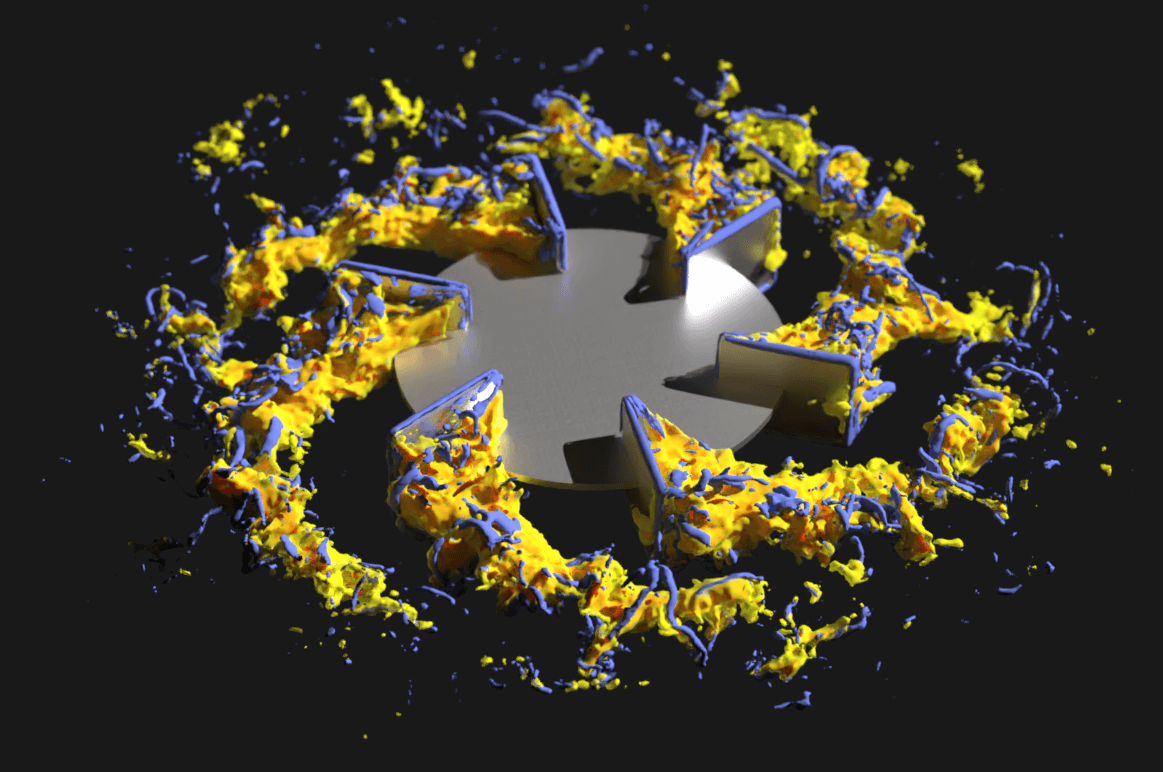
In 2020, the pressure was on for major pharmaceutical companies like Pfizer to rapidly scale-up biologic antibody production processes in order to respond to emerging viruses and variants.
“The only way to take a medicine from the lab to the plant is to scale it up,” said M-Star CFD President John Thomas, PhD.
But with the simulation tools of yesterday, this process tends to be complex, costly, and time-consuming.
To minimize experiments and accelerate production, Pfizer turned to M-Star CFD to develop a physics-based, in-silico modeling approach with digital twins.
“That 1-to-1 mapping using foundational transport physics models allows us to get far better insights into what’s going on inside the process and more immediate comparisons to what we’re seeing inside the lab,” said Thomas. These insights provided Pfizer with a roadmap for process scale-up while reducing the number of experiments needed to predict fluid flow in microscope bioreactors.
To learn more about the approach and results, read the full case study.
 Explore the Scientific R&D Software
Explore the Scientific R&D Software