Chemical Engineering Science Article Supports Demand for Faster, Safer Biopharmaceutical Manufacturing Solutions
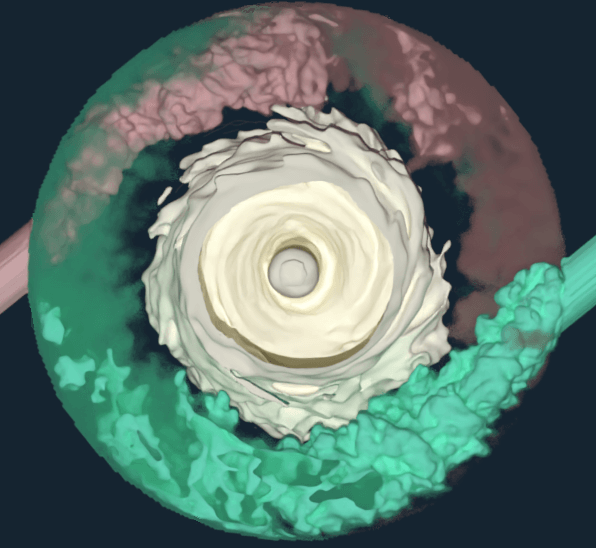
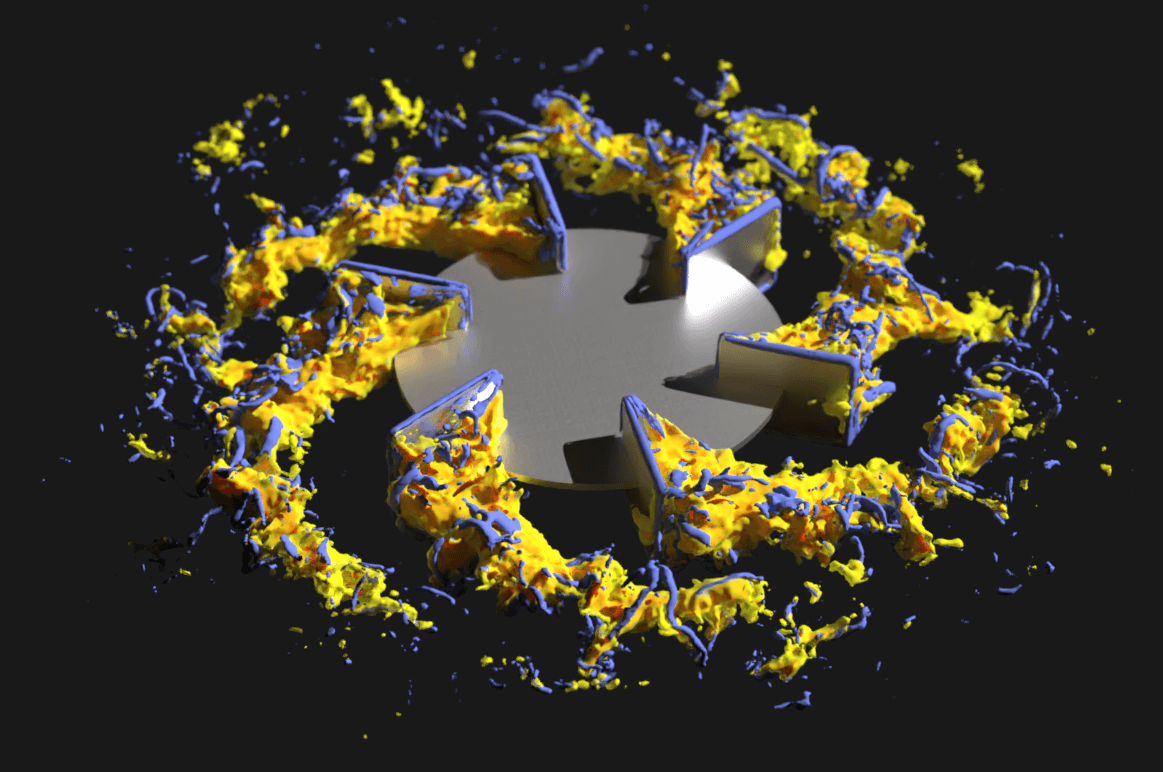
Bioprocess researchers and engineers from M-Star and Pfizer recently published an article in Chemical Engineering Science. The article outlines advanced modeling strategies which helped Pfizer accelerate its vaccine and other drug development process—from product scale-up to tech transfer for contracted production—using M-Star CFD software. The global response to the COVID-19 pandemic triggered an unprecedented demand for a faster, safer, and more flexible biopharmaceutical manufacturing solution.
“The approach we present in the Chemical Engineering Journal article can be utilized as a digital scale-up and tech-transfer strategy for bioreactors used in the biopharma industry,” says John A. Thomas, PhD, president of M-Star Simulations.
“M-Star CFD creates a ‘digital twin’ with computational fluid dynamics to validate things other CFD tools cannot. I am proud that our software helped advance Pfizer’s COVID-19 vaccine and other vaccines and drugs with companies like AbbVie, AstraZeneca, and Bristol Myers Squibb.”
 Explore the Scientific R&D Software
Explore the Scientific R&D Software